Close to 130,000 people globally are affected by cystic fibrosis, a rare genetic disease which leads to respiratory infections and respiratory failure without treatment. Cystic fibrosis (CF) causes thick, sticky mucus to build up in the lungs, digestive tract, and other organs because of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. These mutations prevent chloride from moving to the cell surface, and prevent the movement of water in tissues, which is necessary for preventing the buildup of mucus in CF. When the CFTR protein isn’t working properly, epithelial cells that line the passageways of the lungs, pancreas, and other organs produce mucus that can clog airways and ducts, making it difficult to breathe and digest food. Despite the advancement made in CF with current therapies, up to 15% of patients do not benefit from current therapies.
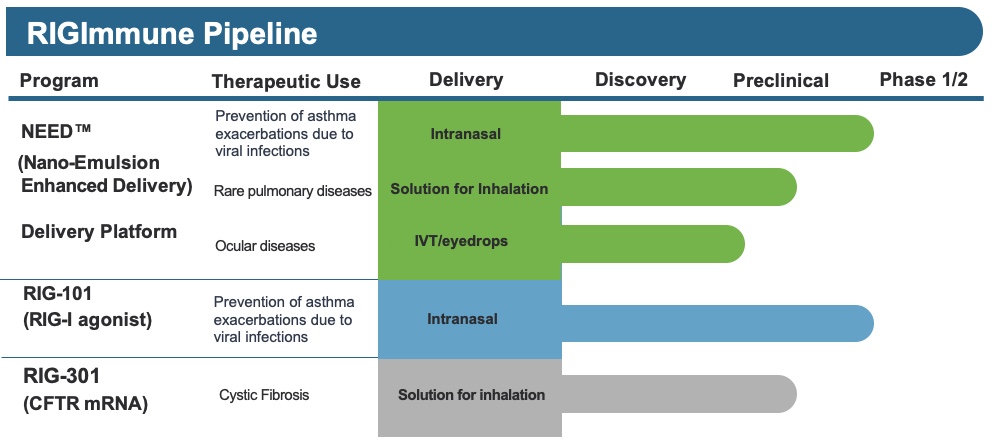
Our second product development candidate is RIG-301, a compound that utilizes the non-LNP NEED™ platform for the targeted delivery of an mRNA payload. RIG-301 is designed to deliver a full-length CFTR protein for the treatment of cystic fibrosis patients, regardless of the specific genetic mutation. RIG-301 could offer a potential effective treatment for a broader range of CF patients as compared to current clinical interventions.
Our innovative inhaled NEED™ formulation enables delivery of RIG-301 directly to the airways affected by cystic fibrosis. This approach effectively navigates the unique challenges of drug delivery in cystic fibrosis lungs, promising enhanced therapeutic efficacy and patient outcomes.